What makes retatrutide different is its “triple action” approach. It mimics three natural hormones that help the body regulate hunger, manage blood sugar and use stored fat for energy.
If you have seen headlines or social posts about it, you are not alone in wondering how it works and what it could mean for future care. Below, we explain how retatrutide works inside the body, when effects tend to begin, and what the early clinical data tells us so far.
Key Takeaways
- Retatrutide acts on three hormone receptors: GLP-1, GIP and glucagon.
- This triple-pathway may increase fat burning and reduce appetite more than current GLP-1 medicines.
- In early trials, average weight loss reached 2–5% by week 4 and up to 24.2% by 48 weeks (12mg dose).
- Reported side effects were mostly mild to moderate, mainly stomach symptoms similar to other GLP-1 medicines.
- Retatrutide is still in clinical testing and not approved in the UK. Buying it online is unsafe and illegal.

Retatrutide’s mechanism of action
Retatrutide’s mechanism of action combines the effects of three hormones naturally produced after eating: glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP) and glucagon.
Let’s look at each of the three receptors that retatrutide acts on and what they do in the body.
GLP-1 receptor
This is the same receptor targeted by Wegovy and Mounjaro. Mounjaro also acts on the GIP receptor.
GLP-1 plays several important roles. It slows how quickly the stomach empties, helping meals feel more satisfying and sends signals to the brain that reduce appetite. After eating, GLP-1 boosts insulin release as glucose rises and suppresses glucagon, which would otherwise raise your blood sugar between meals.
By activating this pathway, retatrutide helps smaller portions feel sufficient, curbs snacking urges and keeps energy levels steady throughout the day.
GIP receptor
GIP helps the body use insulin more effectively after meals, allowing blood sugar levels to settle more smoothly. In the brain, GIP signals can dampen appetite, making it easier to feel satisfied with smaller portions.
The GIP receptor is also activated by Mounjaro.
Glucagon receptor
The glucagon pathway is what sets retatrutide apart from other weight loss injections.
On its own, glucagon usually raises blood sugar. But with retatrutide, the effects of GLP-1 and GIP balance that out. The result appears to be an increase in energy expenditure, meaning the body burns more calories even at rest.
It also promotes the use of stored fat for fuel, may reduce appetite, and early studies suggest it could help reduce liver fat too.
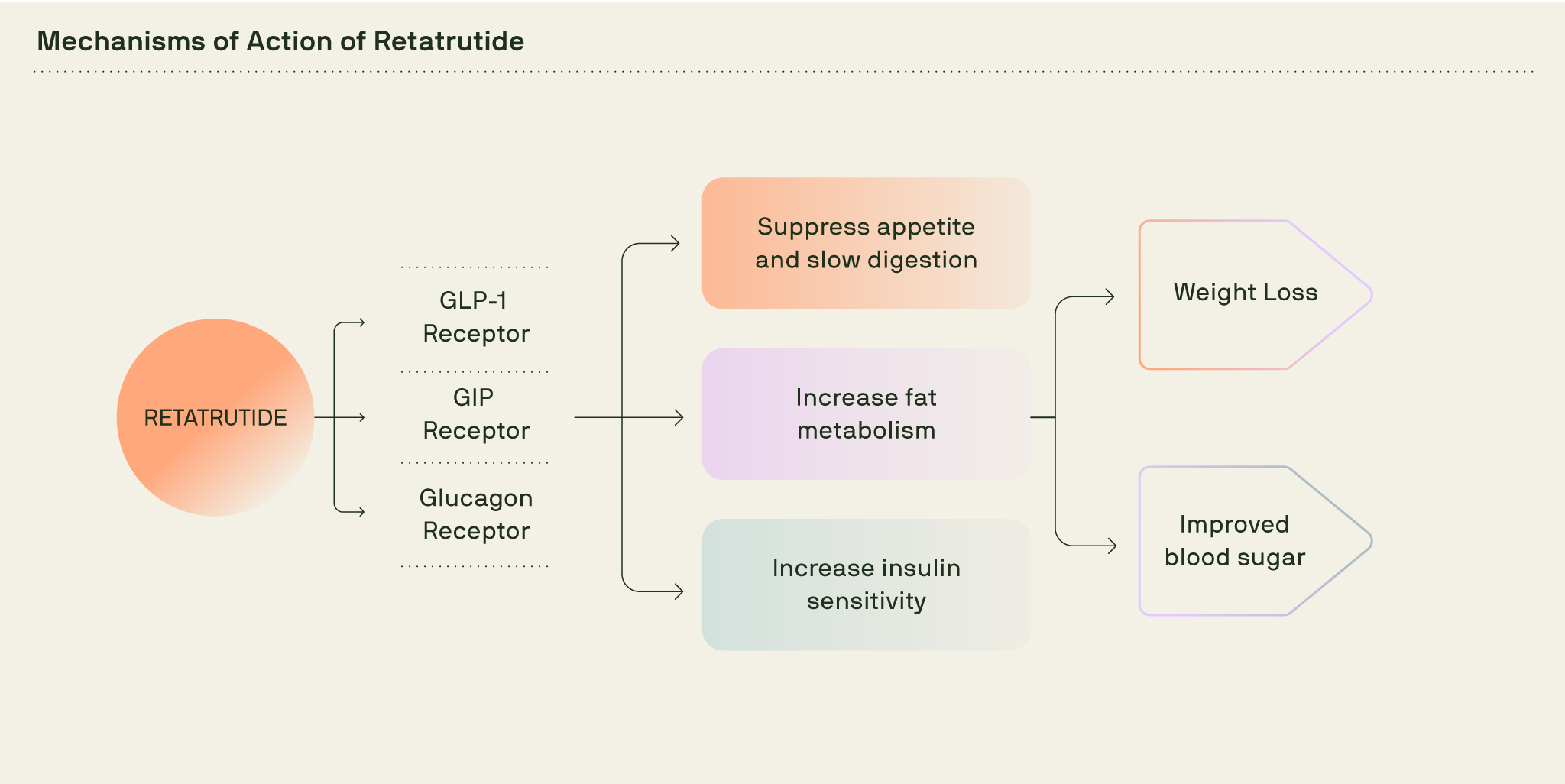
How retatrutide works in the body
After injection, retatrutide circulates and activates three receptors: GLP-1, GIP and glucagon. Together they reduce appetite, smooth post-meal blood sugar spikes, and nudge the body to use more stored fat for energy.
In early trials, people also saw benefits beyond weight loss, including:
- Better blood sugar control (lower HbA1c levels)
- Reduced blood pressure
- Improved cholesterol
- Lower levels of liver fat
To learn more about retatrutide’s wider benefits, see our Retatrutide benefits article
How long before retatrutide works
Retatrutide tends to start working within 2 to 4 weeks. In a Phase 2 study, average weight loss in the first four weeks was about 2–5%, depending on the dose.
Typical pattern in trials (doses 2 mg and above)
As with other weight loss injections, everyone’s pace is different. Some people notice changes within a few weeks, while for others it takes a bit longer. For a deeper look at outcomes over 48 weeks, see our Retatrutide results.
Potential side effects
Reported side effects in studies have been similar to other weight loss injections. These may include:
- Nausea, vomiting or diarrhoea (most common early on)
- Constipation
- Fatigue
- Mild increases in heart rate (similar to changes seen with GLP-1 medicines)
Side effects were more frequent at higher doses (8 mg and 12 mg), but still rated mild to moderate overall.
Availability in the UK
Retatrutide looks like the next step in weight loss treatment, a triple-G injection that could go beyond the limits of current medications. But it is still being studied. Phase 3 trials are ongoing and no version is approved for prescription use in the UK. For now, access is limited to regulated clinical trials.
To explore approved options available today, you can read about Voy’s licensed treatments or check your eligibility now.